DNA Extractions for adult homogenates of Porites Thermal Transplant Batch 2
DNA extractions for Porites Thermal Transplant ADULTS BATCH #2
Goal
To process DNA for Porites astreoides adult homogenates for the Thermal Transplant 2018 experiment using this protocol.
Samples
All samples were snap frozen, then airbrushed with filtered seawater. 0.5 mL of the homogenate was aliquoted then re-frozen at -80 °C.
| Vial | Sample Type | Year | Coral ID |
|---|---|---|---|
| 17-8 | Adult Homogenate | 2017 | P-3 |
| 17-241 | Adult Homogenate | 2017 | R-5 |
| 17-304 | Adult Homogenate | 2017 | R-15 |
| 17-655 | Adult Homogenate | 2017 | R-9 |
| 17-610 | Adult Homogenate | 2017 | P-16 |
| 18-430 | Adult Homogenate | 2018 | P-3-A |
| 18-442 | Adult Homogenate | 2018 | R-9-B |
| 18-454 | Adult Homogenate | 2018 | P-12-A |
| 18-466 | Adult Homogenate | 2018 | R-15-B |
| 18-478 | Adult Homogenate | 2018 | P-16-A |
Sample Preparation and Digestion
- Following the Biological fluids and cell protocol
- Take samples out from -80 °C.
- Immediately added 500uL Biofluid Cell Buffer (red) and 50 μl of Proteinase K to each sample.
- Vortex and spin down.
- Incubate for 30 minutes at 55 °C on 1100 rpm.
- Centrifuge at 8,000 rcf for 30 seconds to remove debris.
- Transfer 1000 μl of the supernatant to a new, labelled 5 ml centrifuge tube.
DNA Extraction
- Add equal volume (1000 uL) of Genomic Binding Buffer to each 5 ml tube
- Finger flick to mix
- Add 700 uL of the 5 mL tube to the spin column
- Centrifuge at 12,000 g (rcf) for 1 minute
- Transfer spin column to a new column (discard previous column)
- Repeat steps 2-3 until the liquid is gone (3x)
- Add 400 uL pre-wash buffer to each spin column
- Centrifuge at 12,000 g (rcf) for 1 minute
- Remove and discard flow through
- Add 700 uL pre-wash buffer to each spin column
- Centrifuge at 12,000 g (rcf) for 1 minute
- Remove and discard flow through
- Add 200 uL wash buffer to each spin column
- Centrifuge at 12,000 g (rcf) for 2 minute
- Transfer spin columns to new 1.5 mL centrifuge tubes
- Add 50 uL of warmed 10mM Tris HCl directly to the filter in the spin column
- Incubate at room temperature for 15 minutes
- Centrifuge at 12,000 rcf for 1 minute
- Add another 50 uL of warmed 10mM Tris HCl directly to the filter in the spin column
- Incubate at room temperature for 5 minutes
- Centrifuge at 12,000 rcf for 1 minute
- Label final tubes
- Aliquot 10 uL into labelled PCR tubes for QC
- Store labelled samples in -20 °C
Quantify Results
Qubit
To test DNA quantity: Qubit
DNA: Broad Range
| DNA (ng/uL) | |
|---|---|
| Standard 1 | 171.62 |
| Standard 2 | 17532.54 |
| 17-8 | 6.60 |
| 17-241 | 37.6 |
| 17-304 | 16.0 |
| 17-610 | 41.8 |
| 17-655 | 40.8 |
| 18-430 | 32.8 |
| 18-442 | 9.94 |
| 18-454 | 34.4 |
| 18-466 | 21.2 |
| 18-478 | 18.8 |
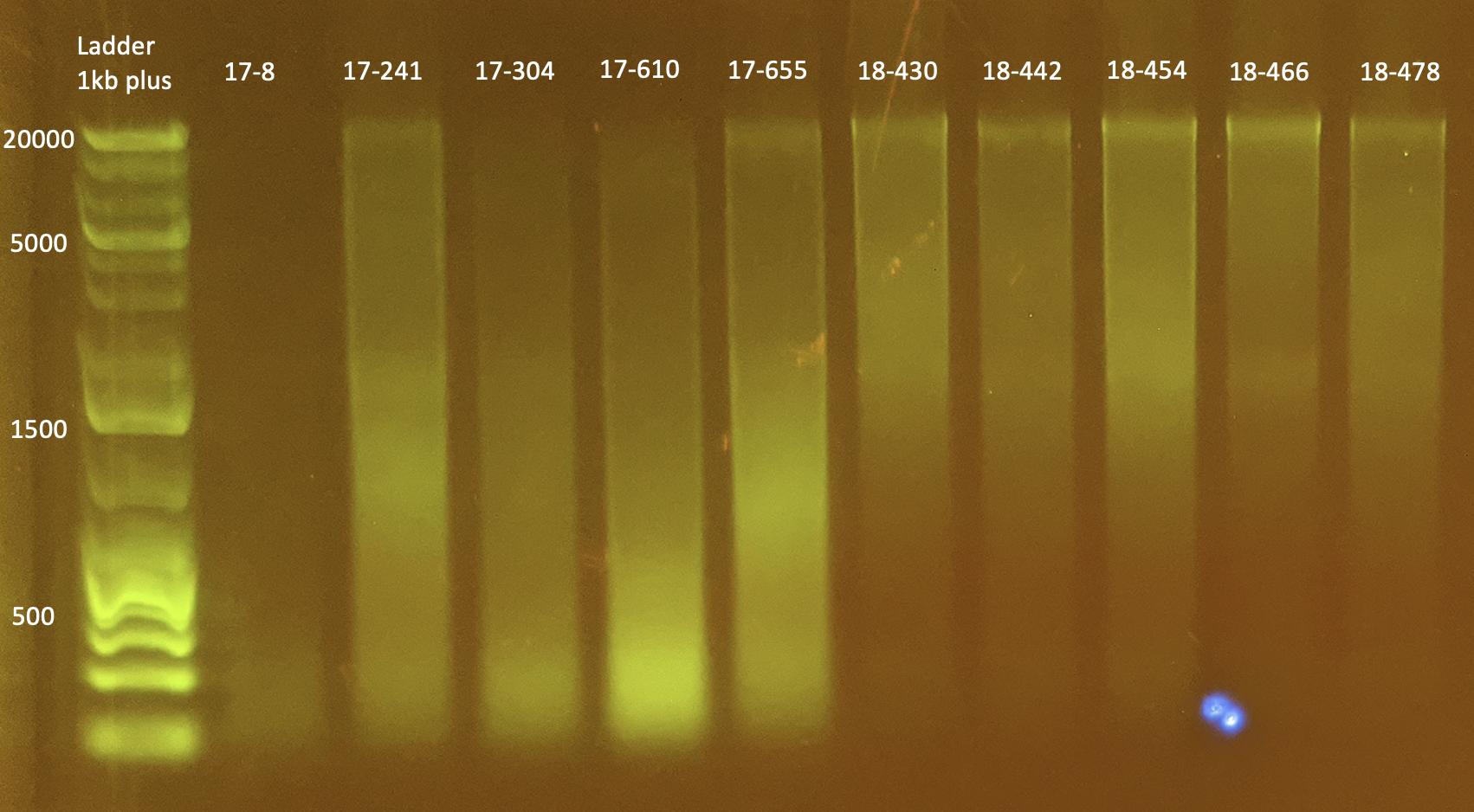
Gel Electrophoresis
To test DNA quality: Gel Electrophoresis
- Today I did a small gel of the above protocol and ran it at 100V.
Written on August 7, 2020
