DNA RNA Extractions Thermal Transplant Porites Trial 2
DNA/RNA extractions for Porites Thermal Transplant Trial #2 - ADULTS
Goal
To stream line the protocol for DNA/RNA extractions for Porites astreoides for the Thermal Transplant 2018 experiment by adding a heating incubation step to digest sample more.
Protocol description
I used a modified version of this protocol from 20191203 extraction.
Samples
I am using the extra supernatant from the sample bead bashed with DNA/RNA shield. Therefore I will have a direct comparison of the extra incubation digestion step. The sample numbers will be the same as 20191203, but the tubes will be labelled “B-#”.
Sample Numbers: 29, 25, 33, 49, 23
Protocol Preparation
Reagents and supplies
- RNAse Away
- RNA/DNA shield
- RNAse-free water
- Proteinase K digestion buffer
- Proteinase K
- Isopropanol
- 10mM Tris HCl pH 8.0 made with RNase-free water
- 10% bleach
- Zymo Duet DNA/RNA Extraction kit buffers: DNA/RNA lysis buffer, DNA/RNA Prep Buffer, DNA/RNA Wash Buffer, DNA Digestion buffer, and DNase I
Equipment
- Rocking oven that can be set at 55 °C
- Thermomixer
- Vortexer
- Qiagen Tissue Lyser: Handbook
- Bead tubes: 2mL 0.5mm glass beads and tubes from Fisher Scientific
- Designated RNAse free space
- Tabletop and larger centrifuges for 1.5 mL capable of 12,000xg
- Clippers
Reagent Preparation
- Add 96 mL 100% ethanol (104 mL 95% ethanol) to the 24 mL DNA/RNA Wash Buffer concentrate before use. DNA/RNA Wash Buffer included with D7003T (Mini Prep Plus Kit) is supplied ready-to-use and does not require the addition of ethanol prior to use. Check kit contents and instructions to confirm prep steps.
- Reconstitute the lyophilized (freeze-dried) DNase I as indicated on the vial prior to use. Mix by inversion. Store frozen aliquots.
- Reconstitute the lyophilized (freeze-dried) 20 mg Proteinase K with 1040 uL Proteinase K Storage Buffer or lyophilized (freeze-dried) 5 mg Proteinase K with 260 uL Proteinase K Storage Buffer. Vortex to dissolve. Store at -20 °C.
Sample Preparation
- Thaw samples from -80 °C.
- Aliquot 300 μl of sample to a new labelled tube.
- 30 μl of Proteinase K digestion buffer (10:1 ratio of sample:digestion buffer), and 15 μl of Proteinase K (2:1 ratio of digestion buffer:Proteinase K).
- Vortex and spin down.
- Incubate at 56 °C for 30 mins @ 600rpm
- Centrifuge at 10,000 rcf for 30 seconds to remove debris
- Transfer 300 μl of the supernatant to a new labelled tube
Zymo Duet RNA DNA Extractions
DNA Extraction
- Set up yellow DNA spin columns and collection tubes, label appropriately
- Warm elution liquids to 70 °C (10mM Tris HCl pH. 8.0 and RNase free water)
- Add equal volume (to supernatant; 300 µl) DNA/RNA lysis buffer to each sample tube
- Finger flick to mix tubes
- Add 600 µl (total volume) of sample gently to the yellow DNA spin column
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Important Save the flow through from this step: transfer to a new 1.5mL tube labeled for RNA
- Add 400 µl DNA/RNA Prep Buffer gently to the yellow DNA spin columns
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Discard flow through (Zymo kit waste)
- Add 700 µl DNA/RNA Wash Buffer gently to the yellow DNA spin columns
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Discard flow through (Zymo kit waste)
- Add 400 µl DNA/RNA Wash Buffer genetly to the yellow DNA spin columns
- Centrifuge at 16,000 rcf (g) for 2 minutes
- Discard flow through (Zymo kit waste)
- Transfer yellow columns to new 1.5 mL microcentrifuge tubes (“E1”)
- Add 50 µl warmed 10mM Tris HCl to each yellow DNA column by dripping slowly directly on the filter
- Incubate at room temperature for 5 minutes. Centrifuge at 16,000 rcf (g) for 30 seconds keep the flow through
- Add another 50 µl warmed 10mM Tris HCl to each yellow DNA column by dripping slowly directly on the filter
- Incubate at room temperature for 15 minutes.
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Throw away filter and keep flow through.
- Aliquot 10 µl of the elution to 0.5 mL PCR tubes for Qubit and Gel Electrophoresis analysis.
- Store at 4 °C if quantifying the same day or the next, if waiting longer store in -20 °C freezer.
RNA Extraction Can do concurrently with DNA Extraction after DNA Extraction Step 7
- Add equal volume (600 µl) 100% EtOH to the 1.5 mL tubes labeled for RNA containing the original yellow column flow through
- Vortex and spin down to mix
- Add 600 µl of that liquid to the green RNA spin columns
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Discard flow through (Zymo kit waste)
- Add 600 µl to the green RNA spin columns (the rest from the 1.5mL RNA tubes)
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Get DNase I from freezer
- Discard flow through (Zymo kit waste)
- Add 400 µl DNA/RNA Wash Buffer gently to each green RNA column
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Discard flow through (Zymo kit waste)
- Make DNase I treatment master mix:
- 75µl DNA Digestion buffer x # of samples
- 5µl DNase I x # of samples
Today I had 5 samples, therefore: 375 µl of DNA Digestion buffer and 25 µl of DNase I
- Add 80 µl DNase I treatment master mix directly to the filter of the green RNA columns
- Incubate at room temp for 15 minutes
- Add 400 µl DNA/RNA Prep Buffer gently to each column
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Discard flow through (Zymo kit waste)
- Add 700 µl DNA/RNA Wash Buffer gently to the green RNA spin columns
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Discard flow through (Zymo kit waste)
- Add 400 µl DNA/RNA Wash Buffer genetly to the green RNA spin columns
- Centrifuge at 16,000 rcf (g) for 2 minutes
- Discard flow through (Zymo kit waste)
- Transfer green columns to new 1.5 mL microcentrifuge tubes
- Add 50 µl warmed DNase/RNase free water to each green RNA column by dripping slowly directly on the filter
- Incubate at room temp for 5 minutes
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Repeat steps 25-27 for a final elution volume of 100 µl
- Aliquot 5 µl of the final elution to 0.5 mL PCR tubes for Qubit and TapeStation analysis.
- Store all tubes in the -80 °C freezer.
Clean-up
- Place tissue and liquid in the waste container labeled Zymo extraction waste.
- Wipe down RNA free area with RNase away and kimwipes.
- Throw away all tips and restock tip boxes if necessary.
Testing Quantity and Quality
To test RNA and DNA quantity: Qubit
To test RNA quality: TapeStation
To test DNA quality: Gel Electrophoresis
Solution Key:
DNA/RNA Lysis Buffer: Contains a high concentration of chaotropic salts, which destabilize hydrogen bonds, van der Waals forces and hydrophobic interactions. This destabilizes proteins, including nucleases. The buffer will also disrupt association of nucleic acids with water.
DNA/RNA Wash Buffer: Used to remove residual proteins and carbohydrates, and purifies the molecular end product.
DNase I: deoxyribonuclease is an enzyme that catalyzes the hydrolytic cleavage of phosphodiester linkages in the DNA backbone, thus degrading DNA. This is used in the RNA extraction step to purify RNA final product.
DNA/RNA Prep Buffer: Ethanol and guanidinium chloride used a deproteinization measure. Guanidinium is a very strong denaturing agent that will dissolve cells and RNAses.
EtOH: the addition of alcohol further enhances and influences the binding of nucleic aicds to the silica (see spin colum description below).
10 mM Tris HCl: DNA is more stable at slightly basic pH and will dissolve faster in a buffer than water. Using Tris instead of water will enhance rehydration of high molecular weight DNA.
DNA/RNase free water: Used as an elution in the RNA extraction steps. RNA can tolerate a slightly acidic pH and dissolves. Warmed water as an elution increases high molecular yield.
Yellow DNA/Green RNA Spin Columns: contain a silica membrane that binds to nucleic acids. Proteins and polysaccharides should be in the flow-through liquid that is discarded while RNA and DNA is kept on the membrane.
Proteinase K: an enzyme that cleaves the peptide bond in proteins next to the carboxyl group of hydrophobic amino acid residues. This decontaminates the sample and leaves purified DNA/RNA.
General workflow of an extraction:
- Cell lysis through disruption of cellular membranes
- Dehydration and precipitation of the cellular proteins (protein denaturation)
- Separation of cellular proteins and other cellular components out of the nucleic acid
- Precipitation and dissolving the nucleic acid
Quanitify Results
Qubit
5 samples + 2 standards + 1 error = 8
DNA Broad Range
199 µl Buffer x 8 = 1592 µl Buffer 1 µl Reagent x 8 = 8 µl Reagent
RNA Broad Range
199 µl Buffer x 8 = 1592 µl Buffer 1 µl Reagent x 8 = 8 µl Reagent
| S1 | S2 | 23 | 49 | 33 | 25 | 29 | |
|---|---|---|---|---|---|---|---|
| DNA | 161.89 | 21813.48 | 5.18 | 2.46 | 9.34 | 2.7 | 2.94 |
| RNA | 382.04 | 10595.29 | 22.8 | 11.8 | 16.6 | 18.0 | 32.8 |
- Initally, I started using the DNA broad range standards (as described in the the 20191203 protocol), however they gave readings of: S1 = 84.50 and S2 = 6972.01. Therefore I used Dr. Puritz’s standards and got the values above.
TapeStation
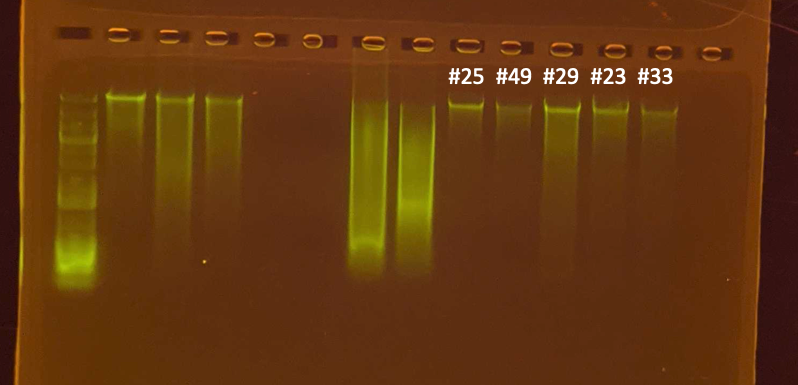
- Sample 33 went on the tape station but it was not read properly.
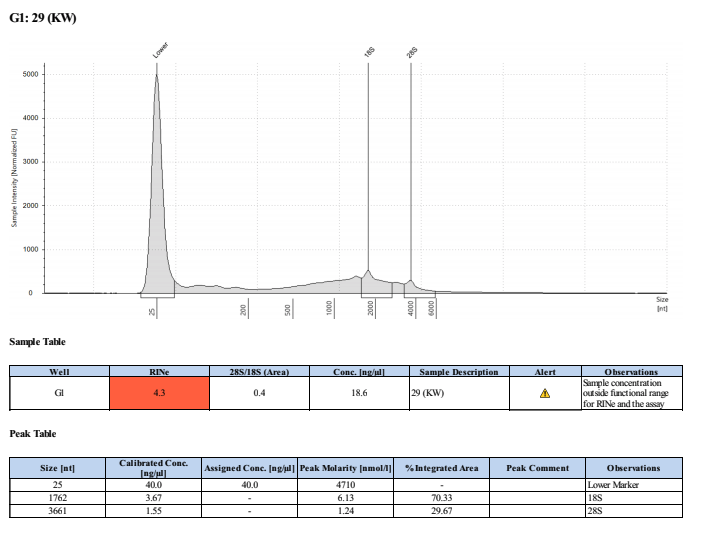
Sample #29:
Sample #49:

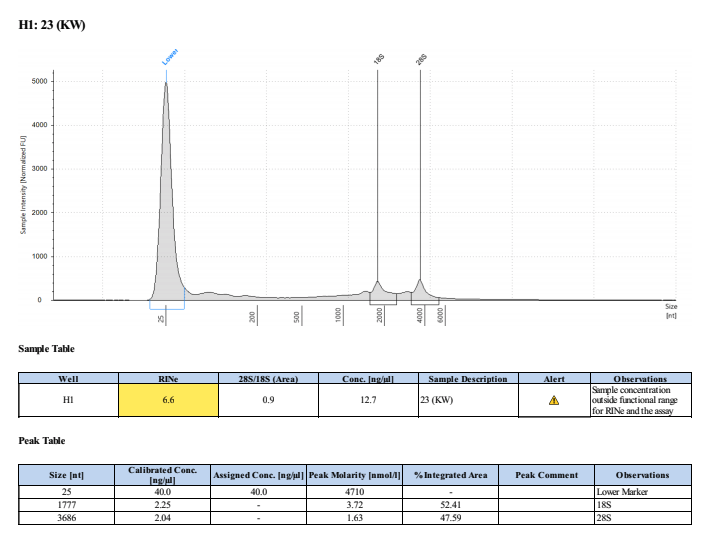
Sample #23:
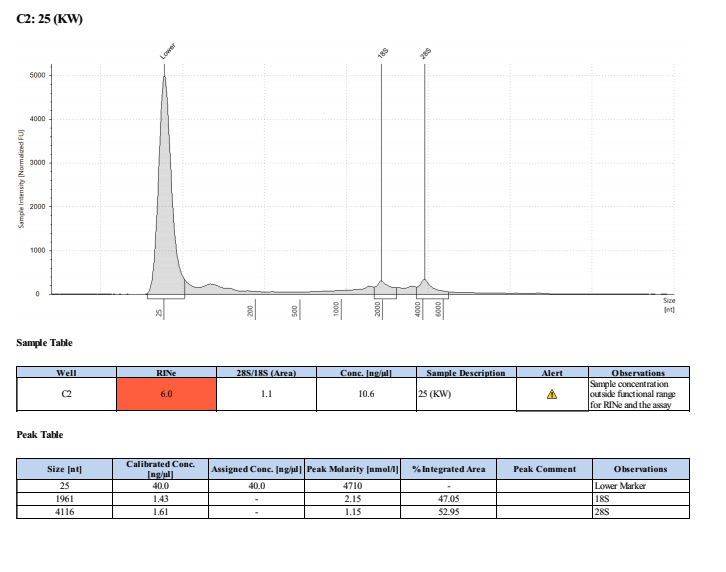
Sample #25:
Gel
Summary of results
- Overall, the incubation step did not increase the yield of DNA or RNA that much. The RNA quality was much worse when incubated. Additionally, it was noted that the final RNA elution was green in tint and the RNA filter in the column was green. This could be the source of error in the tape station results and also contribute to the slight increase in RNA qubit values.
Next steps
- Tomorrow, I will test 2 samples with no heating incubation step. Additionally, I will beat beat for 3-4 minutes and input the entire volume of DNA/RNA shield (~800-900 uL) into the column. Hopefully, this will increase the sample input in the column, therefore increasing the yield and maintaining the RNA quality with no heating step.
