DNA-RNA-Extraxtion-Thermal-Transplant-Porites-Homogenates
Goal
To test the protocol for DNA/RNA extractions for airbrushed homogenates of Porites astreoides for the Thermal Transplant 2017/2018 experiment.
Samples
I used 6 adult P. astreoides samples that were snap frozen, then airbrushed with filtered seawater. 0.5 mL of the homogenate was aliquoted then re-frozen at -80 °C. I used 3 corals from two different time points of the experiment, post treatment (2017) and post-transplant (2018)
Sample Numbers
- 17-26
- P12-T2 (2017: Patch + High Treatment)
- 17-295
- R7-T2 (2017: Rim + Ambient Treatment)
- 17-682
- R19-T2 (2017: Rim + High Treatment)
- 18-19
- R7-B (2018: Rim + Ambient Treatment + Rim Transplant)
- 18-31
- P12-B (2018: Patch + High Treatment + Rim Transplant)
- 18-43
- R19-B (2018: Patch + High Treatment + Rim Transplant)
Protocol description
I used a modified version of this protocol for homogenates.
Homogenate preparation
- Immediately after taking sample out of the -80°C freezer, add 500μL of DNA/RNA shield
- Once fully thawed, add 100 μL of Protenase K digestion buffer (Derived from volume of homogenate and 300/30 ratio from Zymo Protocol) and 50 μL of Protenase K (Derived from volume of homogenate and 300/15 ratio from Zymo Protocol)
- Vortex and spin down.
- Incubate on Thermomixer at 56°C at 1500 rpm for 30 minutes
- Spin down for 30 seconds to pellet any debris
- Remove 1000 μL of the supernatant and transfer in 5 mL tubes. Avoid any debris.
Zymo Duet RNA DNA Extractions
Modified from the Zymo protocol.
DNA Extraction
- Add equal volume of DNA lysis buffer (1000 μL) as sample volume to the 5 mL tube with sample
- Vortex to mix
- Transfer 700 μL into DNA filter column (yellow)
- Centrifuge at 16,000 rcf for 30 seconds
- Remove flow through liquid and transfer into a new 5 mL tube labeled for RNA
- Repeat steps 2 until all liquid is gone (x 3)
- Add 400 μL of DNA/RNA prep buffer to column
- Centrifuge at 16,000 rcf for 30 seconds
- Remove the flow through and transfer to waste
- Add 700 μL of DNA/RNA wash buffer
- Centrifuge at 16,000 rcf for 30 seconds
- Remove the flow through and transfer to waste
- Add 400 μL of DNA/RNA wash buffer
- Centrifuge at 16,000 rcf for 2 minutes
- Remove the flow through and transfer to waste
- Removed column and place into a new sterile 1.5 mL tube
- Add 50 μL of 10 mM Tris HCL (warmed to 55°C) directly to filter
- Incubate at room temperature for 15 minutes
- Centrifuge at 16,000 rcf for 30 seconds
- Keep flow through in tube
- Add another 50 μL of 10 mM Tris HCL (warmed to 55°C) directly to filter
- Incubate at room temperature for 5 minutes
- Centrifuge at 16,000 rcf for 30 seconds
- Discard column and keep flow through in tube
- Aliquot 10 μL into PCR tubes for QC.
- Store remaining aliquot at -20°C
RNA Extraction
- Add equal volume of 100% Ethanol (2000 μL) as sample volume to the 5 mL tube with sample
- Vortex to mix
- Transfer 700 μL into RNA filter column (green)
- Centrifuge at 16,000 rcf for 30 seconds
- Remove flow through liquid and transfer into a new 5 mL tube labeled for Protein
- Repeat steps 12 until all liquid is gone
- Add 400 μL of DNA/RNA wash buffer to column
- Centrifuge at 16,000 rcf for 30 seconds
- Remove the flow through and transfer to waste
- Make DNase I master mix: [75μL DNA digestion buffer and 5μL DNase] X n (sample #)
Today I had 6 samples therefore: 450 μL of DNA digestion buffer and 30 μL of DNase I
- Add 80μL of DNase I master mix directly to the filter of each column. Incubate at room temperature for ~15 minutes
- Add 400 μL of DNA/RNA prep buffer to column
- Centrifuge at 16,000 rcf for 30 seconds
- Remove the flow through and transfer to waste
- Add 700 μL of DNA/RNA wash buffer
- Centrifuge at 16,000 rcf for 30 seconds
- Remove the flow through and transfer to waste
- Add 400 μL of DNA/RNA wash buffer
- Centrifuge at 16,000 rcf for 2 minutes
- Remove the flow through and transfer to waste
- Removed column and place into a new sterile 1.5 mL tube
- Add 50 μL of RNAase-free water (warmed to 55°C) directly to filter
- Incubate at room temperature for 5 minutes
- Centrifuge at 16,000 rcf for 30 seconds
- Keep flow through in tube
- Made the gel during this incubation
- Add another 50 μL of RNAase-free water (warmed to 55°C) directly to filter
- Incubate at room temperature for 5 minutes
- Centrifuge at 16,000 rcf for 30 seconds
- Discard column and keep flow through in tube
- Took out qubit standards
- Aliquot 5 μL in PCR strip tubes for QC.
- Store remaining aliquot at -80°C
Clean-up
- Place tissue and liquid in the waste container labeled Zymo extraction waste.
- Wipe down RNA free area with RNase away and kimwipes.
- Throw away all tips and restock tip boxes if necessary.
Testing Quantity and Quality
Quantify Results
Qubit
To test RNA and DNA quantity: Qubit
6 samples + 2 standards + 1 error = 9
DNA Broad Range
199 µl Buffer x 9 = 1791 µl Buffer 1 µl Reagent x 9 = 9 µl Reagent
RNA Broad Range
199 µl Buffer x 9 = 1791 µl Buffer 1 µl Reagent x 9 = 9 µl Reagent
| Sample | DNA (ng/uL) | RNA (ng/uL) |
|---|---|---|
| Standard 1 | 201.52 | 396.07 |
| Standard 2 | 21429.55 | 9327.86 |
| 17-26 | 10.3 | 24.8 |
| 17-295 | 21.6 | 34.6 |
| 17-682 | 22.8 | 28.6 |
| 18-19 | 16.2 | 30.2 |
| 18-31 | 6.36 | 10.0 |
| 18-43 | 17.0 | 22.6 |
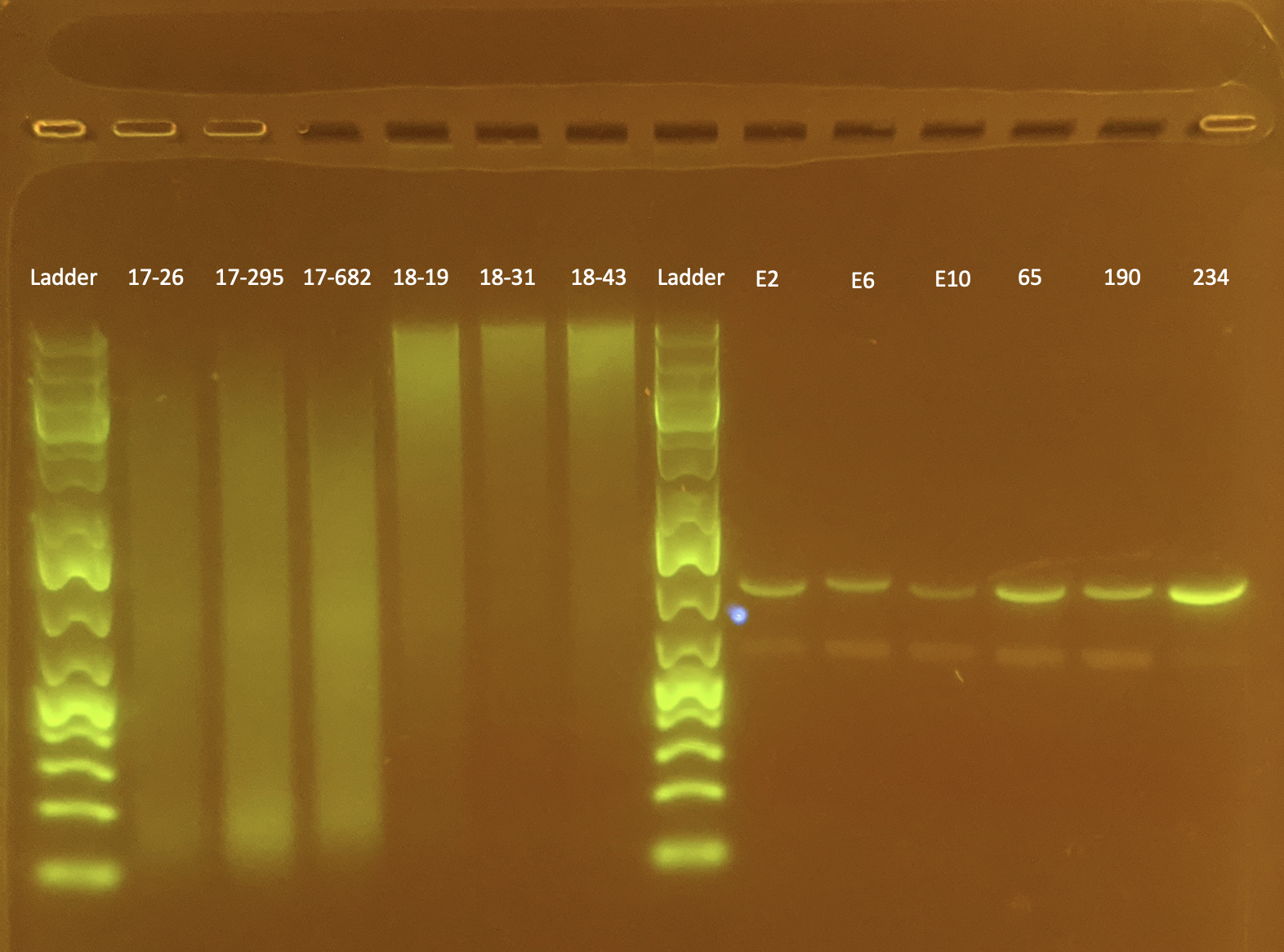
Gel Electrophoresis
To test DNA quality: Gel Electrophoresis
- Today I did a small gel of the above protocol and ran 6 samples for MS
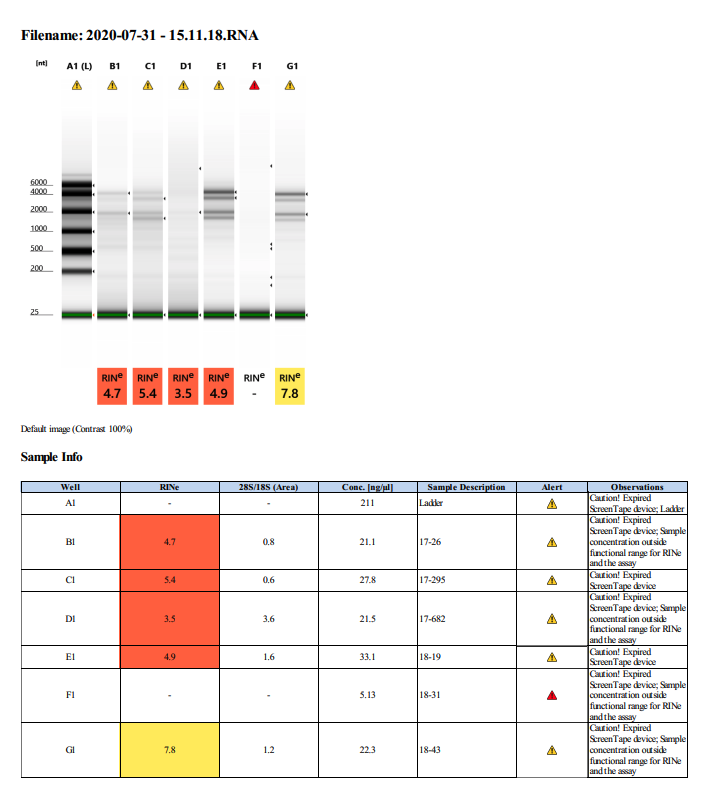
TapeStation
To test RNA quality: TapeStation
Overall Summary:
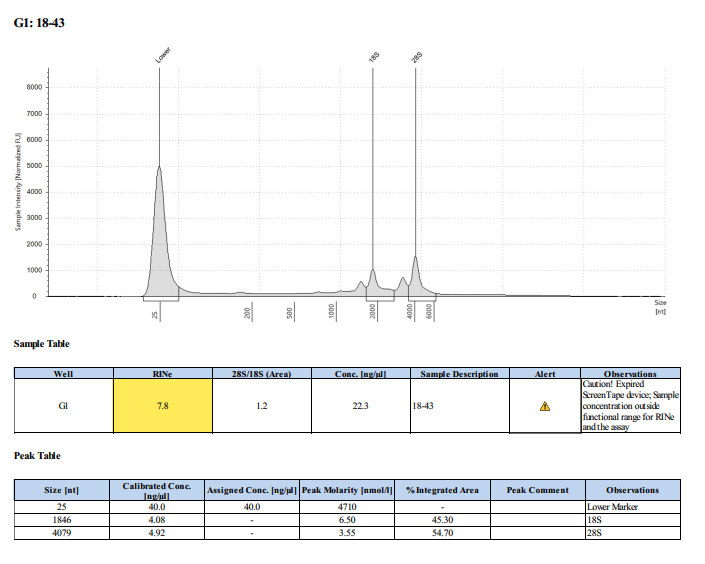
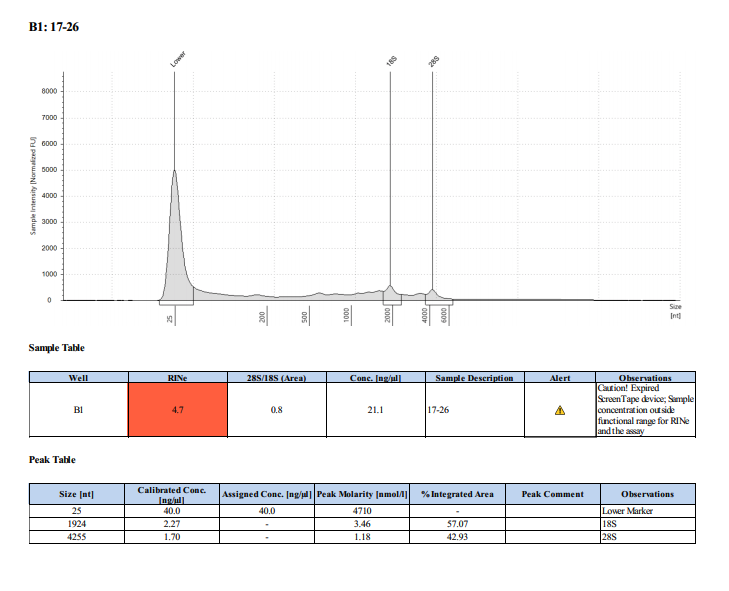
Sample #17-26:
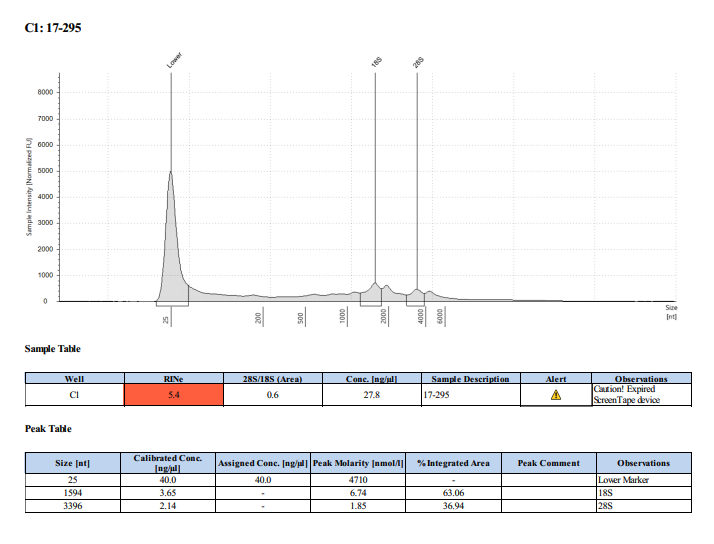
Sample #17-295:
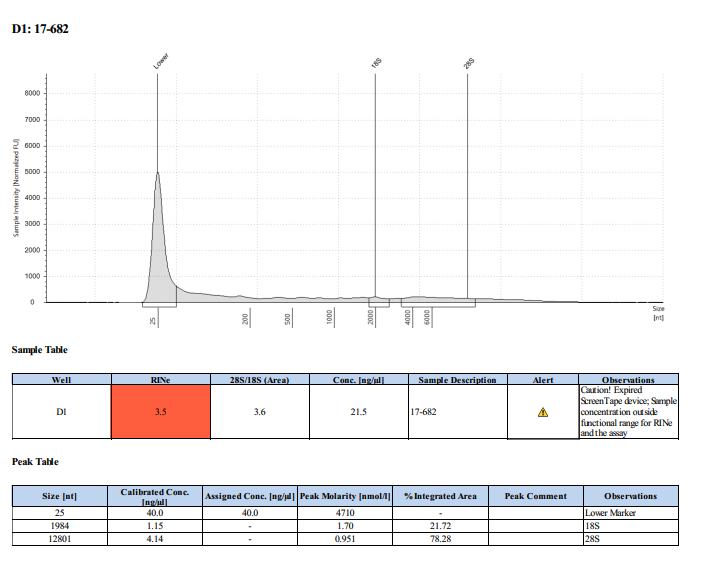
Sample #17-682:
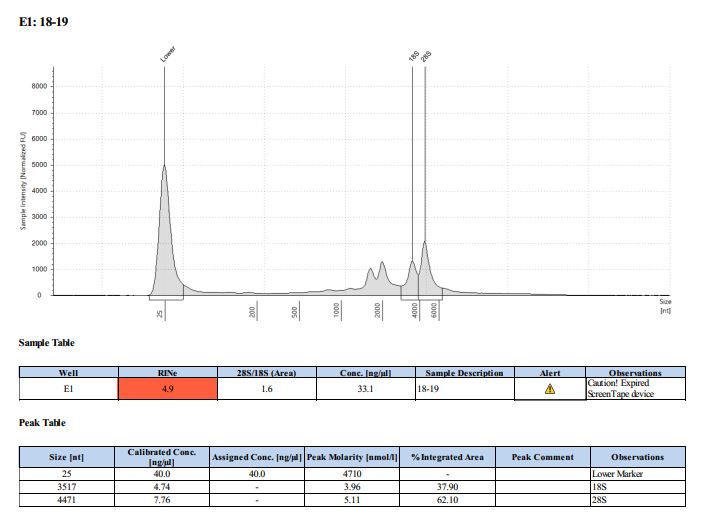
Sample #18-19:
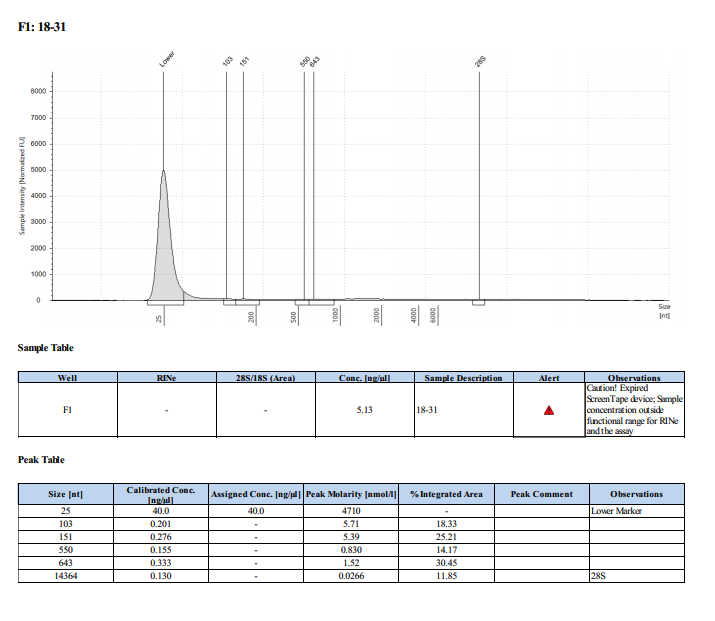
Sample #18-31:
Sample #18-43: